Exactech® Ankle Recall Lawyers
The purpose behind ankle replacement surgery is to improve a patient’s life by relieving pain and giving them full mobility again by repairing their joint damage. Unfortunately, many of these ankle implants have been recalled due to a packaging error that poses a significant risk to patients by causing components to prematurely fail. Exactech® ankle implants such as the VANTAGE® have been recalled as a direct result of this error and patients who have received these implants may be entitled to compensation.
Di Pietro Partners has decades of experience as a medical device litigation firm. Our attorneys work with top quality board certified physicians to properly assess each individual case and ensure the best outcome possible for our clients. If you or someone you know has received an Exactech® ankle implant, it is critical that you seek both legal and medical advice as soon as possible.
To learn more about the Exactech® ankle recall lawsuit and how we can help you with your case, contact our office today. Our attorneys will provide a free case evaluation and pursue claims anywhere in the United States.
Our Product Liability Lawyers on TV
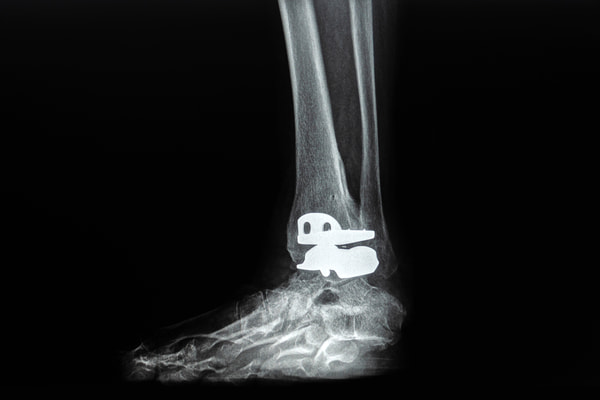
Recalled Exactech® Ankle Implants
VANTAGE® – The Vantage® ankle is a popular ankle replacement device that has been implanted for years in a wide range of patients. It is designed with two different shape options in order to be adaptable to any particular bone shape or condition which makes them highly effective.
Ankle Replacement Revision Surgery
This extensive recovery period can cause serious problems for patients in their daily lives, often requiring them to hire home assistants in order for them to be able to complete daily tasks. The challenges associated with this recovery period usually require financial compensation to cover at home assistant wages as well as lost income and a reduced quality of life.
As discussed previously, the majority of situations involving ankle implant recalls will require hip revision surgery to correct. Because the polyethylene insert is the component that fails as a result of the packaging defect, it requires complete replacement. Although a second ankle surgery is never a welcome experience, the manual replacement of this defective component is a required process in order to rectify the problem that is covered under this particular Exactech® recall.
Exactech® Ankle Replacement Lawsuit
There is currently a recall for Exactech® Vantage total ankle systems by the FDA for defects related to material deterioration caused by improper packaging from the factory. This implant is now listed as part of a Class 2 Device Recall by the Food and Drug Administration (FDA). If you or a loved one are suffering complications that are a result of one of these medical implant devices, there is a strong possibility that you could be eligible to file a lawsuit and receive just compensation.
A Class 2 Device Recall is specifically defined by the Food and Drug Administration as “a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences.” According to this definition, this specific lawsuit may qualify as a class action lawsuit or mass tort.
In order to be eligible for an Exactech® hip replacement lawsuit in the United States, there are two requirements that must be met. Specifically, you or your loved one must have:
- Had an ankle implant surgery using an Exactech® ankle system in the United States
- Suffered from the side effects of a Exactech® ankle implant that is defective or has prematurely degraded
The experienced ankle implant litigation lawyers at Di Pietro Partners can file this lawsuit on your behalf at no cost to you if you meet these qualifications.
About the Manufacturer
Exactech® is a large international orthopedic device manufacturer that’s based in Gainesville Florida. Founded in 1985, their primary market is the United States with over 30 different markets across the world in Europe, Latin America, Asia and the greater Pacific region. With an annual revenue of approximately $225 Million and around 1000 on-site employees, they are a major player in the joint replacement marketplace.
In addition to orthopedic devices, they also design, manufacture and sell surgical instrumentation as well as offer their expertise in the biological, orthobiologic, biotech and computer-assisted surgical fields. The company has grown exponentially since their first offering of a knee replacement device in 1994, with a boost in sales afterwards thanks to their entrance into the shoulder and ankle replacement markets.
Frequently Asked Questions
Q. Which Exactech® ankle implants have been recalled?
Even though there hasn’t been a complete recall on the tire ankle replacement system, the liners used in these implants have been recalled due to improper packing causing premature degradation of these liners. This primarily involves the VANTAGE® series of devices. While the metal portion of these implants are not directly affected by the recall, they ultimately end up suffering from the same long term issues that caused the recall in the first place, which is premature joint wear and failure
Q. What does it cost to file an Exactech® ankle replacement lawsuit?
There are no costs associated with filing a lawsuit through Di Pietro Partners if you or a loved one has been affected by a recalled Exactech® ankle replacement implant device. Other firms may charge for filing before securing settlement but our firm does not charge whatsoever until we have secured a settlement on your claim.
Q. How long do I have to file a Exactech® ankle replacement lawsuit?
Even though the recall was issued in August of 2021, it covers any Exactech® ankle replacement devices dating back to 2004. It should be noted that there is a limited time window in which this can be filed in. For example, Florida statutes clearly outline that victims have a four year window after they know or suspect that their ankle replacement device is defective to file their ankle implant lawsuit. This timeframe can be subjective, which makes it therefore critical to hire the best possible medical device recall attorney in order to ensure that your case is properly represented and you get fair compensation.
Q. What are the settlement amounts on Exactech® ankle lawsuits?
Although this recall is still too new to have any finalized verdicts or settlements on the Exactech® artificial ankle replacement lawsuits, average settlement amounts can be estimated based on amounts awarded in prior mass tort cases involving defective hip implants. Based on our firm’s experience in these cases, the average settlement amount paid out for faulty Exactech® ankle replacement devices should be around $250,000. It should be noted that there are other variables that may impact the value of each individual case.
Q. Who is eligible to receive compensation?
Any individual who has received an Exactech® ankle implant in the United States and is suffering health complications as a result of this device may be entitled to receive compensation from the manufacturer. For any cases where the device was received out of the U.S. we suggest consulting an attorney licensed to practice in that respective country.