DEFECTIVE MEDICAL DEVICES
We rely on doctors, surgeons, and specialists to maintain and protect our health. As patients, we trust their opinions as they’re generally qualified by years of schooling and medical training. For instance, when undergoing a procedure such as hip replacement surgery, we trust it will improve overall quality of life. Unfortunately, certain manufacturers produced defective medical devices which led to serious complications, and in some cases, death. What’s more troubling is many manufacturers produced, marketed, and sold devices while aware of high failure rates. To make matters worse, medical professionals and patients were often never informed of these risks.
As a result, The law firm of Di Pietro Partners is pursuing cases against certain manufacturers whose products cause health complications in patients. As AV Preeminent® rated trial attorneys that work with board certified, medical professionals, we understand your legal and medical rights. Therefore, if you’ve been injured anywhere in the United States by one of the devices below, call our offices for a free and confidential case review. 1.800.712.8462
Acumatch Hip Recall
The purpose behind hip replacement surgery is to improve a patient’s life by relieving pain and giving them full mobility again by repairing their joint damage. Unfortunately, many of these hip implants have been recalled due to a packaging error that poses a significant risk to patients by causing components to prematurely fail. Acumatch® hip implants have been recalled as a direct result of this error and patients who have received these implants may be entitled to compensation.
Exactech Ankle Replacements
The purpose behind ankle replacement surgery is to improve a patient’s life by relieving pain and giving them full mobility again by repairing their joint damage. Unfortunately, many of these ankle implants have been recalled due to a packaging error that poses a significant risk to patients by causing components to prematurely fail. Exactech ankle implants such as the VANTAGE® have been recalled as a direct result of this error and patients who have received these implants may be entitled to compensation.
Exactech Knee Replacements
Knee replacement surgery intends to improve a patient’s life by relieving pain or joint damage. Unfortunately, many knee implants have been recalled due to faulty design and post a significant risk to patients. Exactech knee implants such as OPTETRAK®, OPTETRAK Logic®, TRULIANT®, and VANTAGE® have recently been recalled and patients who received these implants may be entitled to compensation.
Exactech Hip Replacements
Hip replacement surgery intends to improve a patient’s life by treating problems such as arthritis, pain, and loss of mobility. Unfortunately, in recent years, many hip implants have caused serious health complications. Exactech hip implants such as Acumatch, MCS and Novation implants have recently been recalled and patients who received these implants may be entitled to compensation.
MCS Hip Replacements
Hip replacement surgery intends to improve a patient’s life by treating problems such as arthritis, pain, and loss of mobility. Unfortunately, in recent years, many hip implants have caused serious health complications. MCS hip implants have recently been recalled and patients who received these implants may be entitled to compensation.
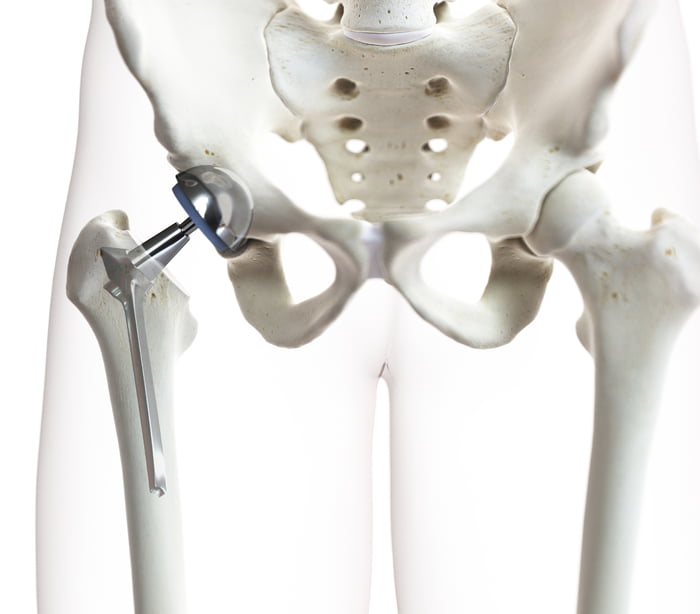
Metal Hip Replacements
Hip replacement surgery intendeds to improve a patient’s life by treating problems such as arthritis, pain, and loss of mobility. Unfortunately, in recent years, many hip implants have caused serious health issues. These issues include: pain, infection, organ failure, high cobalt/chromium levels in the bloodstream, etc. Additionally, complications may lead to a second surgery (Hip Replacement Revision Surgery). Many of these defective medical devices have been recalled and reported by the FDA.
 Specific manufacturers we’re pursuing cases against include:
Specific manufacturers we’re pursuing cases against include:
Smith & Nephew Hip Replacements
Novation Hip Replacements
Hip replacement surgery intends to improve a patient’s life by treating problems such as arthritis, pain, and loss of mobility. Unfortunately, in recent years, many hip implants have caused serious health complications. Novation hip implants have recently been recalled and patients who received these implants may be entitled to compensation.
Optetrak® Knee Replacements
Knee replacement surgery is intended to improve the quality of life for patients who have knee problems such as arthritis, pain, and loss of mobility. Unfortunately many of these knee replacements have caused serious complications in recent years. Manufactured by Exactech, the Optetrak® knee implants have been recently recalled and patients who received these implants may be entitled to compensation.
Vantage® Ankle Replacements
The purpose of ankle replacement surgery is intended to improve a patients quality of life and restore normal function by fixing problems such as arthritis, joint pain and loss of mobility. Unfortunately however, some of these ankle implants have been causing serious complications in recent years in those who have received them. The Vantage® ankle implants have been recalled recently by the manufacturer Vantage® and patients who have received these implants may be entitled to just compensation.
Vantage® Knee Replacements
The purpose of knee replacement surgery is intended to improve a patients quality of life and restore normal function by fixing problems such as arthritis, joint pain and loss of mobility. Unfortunately however, some of these knee implants have been causing serious complications in recent years in those who have received them. The Vantage® knee implants have been recalled recently by the manufacturer Vantage® and patients who have received these implants may be entitled to just compensation.
Inferior Vena Cava Filters (IVC Filters)
Inferior Vena Cava Filters are commonly referred to as “blood clot filters.” These medical devices are supposed to save a patient’s life by preventing a blockage of an artery in the lungs (pulmonary embolism). Sadly, many IVC Filters are prone to failure; thus, these defective medical devices put patients at an unnecessary risk for serious injury or death.

Specific manufacturers we’re pursuing cases against include:
Knee Replacement Devices
Knee Replacement Surgery is supposed to reduce this pain and help patients walk; however, several devices are failures. One of the most common reasons for failure involves the tibial plate loosening. Defective medical devices for knee replacements lead to serious health concerns

Specific devices we’re pursuing cases against include:
The DePuy Synthes Attune Knee System
Shoulder Replacement Devices
Our lawyers fight for those who have fallen victim to a faulty shoulder replacement device that has caused additional injury, or necessitated shoulder replacement revision surgery.