Vantage® Knee Recall Lawyers
Knee replacement surgery is intended to improve a patients quality of life by restoring the normal function of their joint and fixing issues such as joint pain, arthritis and loss of mobility. While most operations are successful in accomplishing these goals, unfortunately there are times when things go wrong with the implants used. In fact, this has become an issue with the Vantage® knee implants which have recently been recalled by the manufacturer Exactech and just compensation may be available to patients who have received these implants.
As an experienced product liability law firm, Di Pietro Partners has many years under their belt successfully getting results for their clients. Our attorneys work directly with highly rated board certified physicians in order to specifically assess each individual case and ensure that the best possible outcome is achieved. If yourself or someone you know is experiencing complications or issues resulting from a Vantage® knee replacement, it is vital to seek out assistance from legal and medical professional experts as soon as possible.
For more information regarding the Vantage® knee recall lawsuit, please reach out to our office today for a free evaluation of your case. We pursue claims anywhere within the United States.
Our Product Liability Lawyers on TV
Vantage Knee Implant Complications
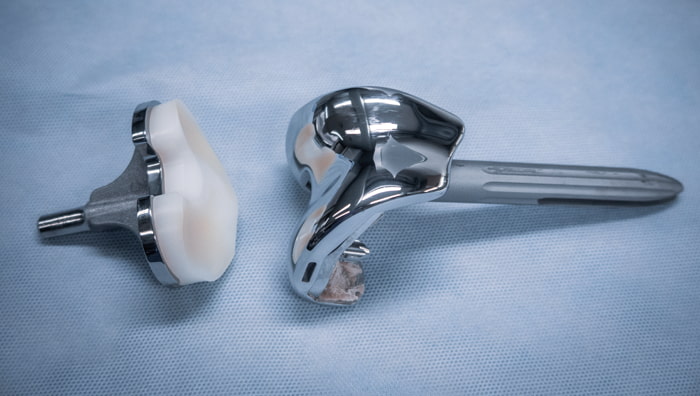
While the majority of Vantage® knee inserts sold and installed over the past several decades have been successful and trouble free, unfortunately there are certain situations where these Exactech manufactured implants have resulted in complications for recipients. This has been traced directly back to defective packaging that allowed implants to be exposed to the atmosphere, causing premature degradation of the material and subsequent joint looseness and failure.
In most cases the best way to rectify the situation is through surgery in order to physically replace any defective parts. Those who are having complications with Vantage® knee inserts normally experience the following symptoms:
- Instability in Joints
- Swelling in the Joint
- Impairment of Joint Function
- Pain in the Joint
- Stiffness in the Joint
Vantage Knee Replacement Lawsuit
There are multiple types of implants manufactured by Exactech that are now being recalled by the FDA. This includes Vantage® implants, which are part of a Class 2 Device Recall by the Food and Drug Administration (FDA). The reason these implants are being recalled is due to material deterioration caused by improper packaging from the factory, which includes a number of other OPTETRAK® and TRULIANT® systems. If you are suffering from complications or issues resulting from a Vantage® insert or any other type of Exactech® medical device, you could be eligible to file a lawsuit and receive just compensation.
Specifically defined by the Food and Drug Administration as “a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences”, a Class 2 Device Recall allows for victims to seek compensation through legal action. Accordingly, this lawsuit may qualify as a mass tort or class action lawsuit.
In order for an individual to be eligible for an Exactech® knee replacement lawsuit in the United States, they must meet several requirements:
- Received a Vantage® knee implant within the United States
- Suffered ill effects from a Vantage® implant that prematurely malfunctioned due to defects
The expert knee replacement litigation attorneys at Di Pietro Partners can file this lawsuit on your behalf at no cost to you. Contact our office today to get started.